2021.04.08
Identification of amino acids involved in monoterpene actions on transient receptor potential (TRP) channels
SOKENDAI Publication Grant for Research Papers program year: 2020
Physiological Science Nguyen Thi Hong Dung
生理科学コース
Structural basis for promiscuous action of monoterpenes on TRP channels
journal: Communications Biology publish year: 2021
DOI: 10.1038/s42003-021-01776-0
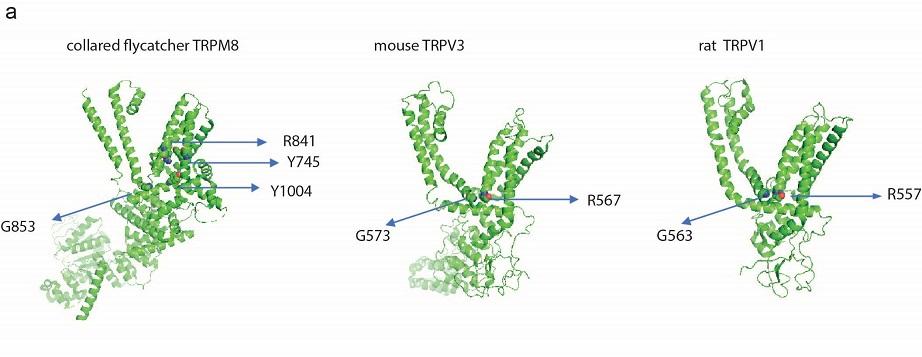
Two amino acid residues arginine (R) and glycine (G) are involved in monoterpenes (menthol and camphor) action located in the linker of transmembranes 4 and 5 of rat TRPV1 (arginine residue at 557 - R557 and glycine residue at 563 - G563) and mouse TRPV3 (arginine residue at 567 - R567 and glycine residue at 573 - G573). We selected rat TRPV1 and mouse TRPV3 because their structures were validated by several previous studies. Although these amino acids are conserved in TRPV1 and TRPV3, the involvement of these amino acids differed between TRPV3 and TRPV1 for chemical-induced and heat-evoked activation. In rat TRPV1, R557 and G563 are involved in menthol and camphor-evoked activation, and R557 and G563 are important for heat-evoked activation. On the other hand, In mouse TRPV3, R567 is involved in both ligand binding and heat-evoked channel activation while R567 is involved in channel gating.
Monoterpenes are major constituents of plant-derived essential oils and have long been widely used for therapeutic and cosmetic applications. The monoterpenes menthol and camphor are activators or blockers for several TRP channels such as TRPV1 and TRPV3. TRPV1 and TRPV3 are crucial for sensing the temperature and natural compounds. The promiscuousness of monoterpenes makes it an important research tool to characterize physiological function and biophysical properties of ion channels. However, the potential of monoterpenes as lead compounds for drug design remains limited because their target regions of action in TRPV1 and TRPV3 remain poorly understood. In this study we identified conserved arginine and glycine residues in the linker of transmembranes 4 and 5 of rat TRPV1 and mouse TRPV3 that are related to the action of these chemicals. We validated these findings with molecular dynamics (MD) simulations. MD simulation data strongly support the involvement of the amino acids involved in mornoterpene actions at an atomic level. MD simulation is a computer-based simulation method for analyzing the physical movements of atoms and molecules. Our findings about the structural basis of monoterpene action contribute to principles of TRP channel regulation by small compounds. Identification of the monoterpenes-binding sites for TRPV1 and TRPV3 presented in this study can serve as a spring board for the design of drugs targeting inflammatory skin conditions, itch, pain, and cancer.
Bibliographic information of awarded paper
- Title: Structural basis for promiscuous action of monoterpenes on TRP channels
- Author name: Thi Hong Dung Nguyen, Satoru G. Itoh, Hisashi Okumura, *Makoto Tominaga
- Journal title: Communications Biology 4: 293
- Publication year:2021
School of Life Science Department Physiological Sciences, Nguyen Thi Hong Dung
My name is Nguyen Thi Hong Dung and I come from Vietnam. I graduated from Da Nang University of Science and Technology, Vietnam with Bachelor Degree. After that, I moved to Japan to continue my study. I got Master Degree from Okayama University and Ph.D. from The Graduate University for Advanced Sciences. Currently, I am a Postdoctoral Researcher in Division of Cell Singling, National Institute for Physiological Sciences.
