2024.12.18
Synthesis and Reactivity Studies of Dendralenes for Chemically Recyclable Copolymers
SOKENDAI研究派遣プログラム 採択年度: 2024
服部修佑
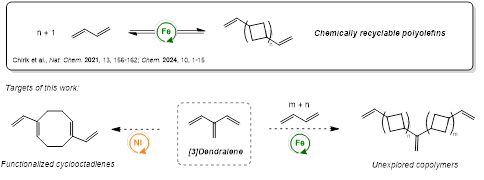
Dendralenes are of high interest as starting materials for synthesizing hydrocarbon products for applications in fuels or chemically recyclable polyolefins.
The development of plastics is an indispensable component of modern life, whereas the features also present a challenge for waste management, which ends up either in landfill or the environment. In this work, we aimed to synthesize the monomer compound, which has the potential to develop a new chemically recyclable polymer material.
Polyolefins are the most abundant group of plastics and essential for many applications, but their inertness hampers degradation and leads to accumulation in the environment. Mechanical recycling is insufficient to enable a circular economy, as it leads to quality loss of the material. An alternative approach, chemical recycling transforms the polymer back to its monomeric units, enabling purification of the monomer and synthesis of pristine, recycled polyolefin. The high thermodynamic stability of commonly used polyolefins makes chemical recycling highly challenging and demands for accessing alternative materials. In this context, the Chirik group has established a new microstructure of butadiene (divinyloligocyclobutane, DVOCB) arising from a sequence of iron-catalyzed [2+2] cycloadditions: This discovery allows for the synthesis of oligomeric and polymeric materials that are fully chemically recyclable and derive exclusively from feedstock hydrocarbons.1, 2 However, the scope of monomers and thus the range of accessible materials remains limited.
In this work, the use of vinyl functionalized butadiene monomers (dendralenes) in the copolymerization of butadiene was targeted. The additional vinyl group of dendralenes represents a potential internal trigger point for chemical recycling, functionalization, or crosslinking of DVOCB and therefore is of high interest to synthesize new materials with distinct properties. In a more general way, dendralenes are only scarcely explored for accessing new hydrocarbon structures that are of interest for applications like fuels. A variety of dendralenes was synthesized successfully and their reactivity with a series of 3d transition metal catalysts explored. However, the strong tendency to form Diels-Alder products already in absence of catalysts makes their handling highly challenging. Future research efforts will therefore continue to identify protocols that allow for synthesizing new hydrocarbon structures from dendralenes.
(1) Mohadjer Beromi, M.; Kennedy, C. R.; Younker, J. M.; Carpenter, A. E.; Mattler, S. J.; Throckmorton, J. A.; Chirik, P. J. Iron-catalysed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes. Nat. Chem. 2021, 13 (2), 156-162.
(2) Nie, C.; Maguire, S. M.; Zheng, C. W.; Mohadjer Beromi, M.; Register, R. A.; Priestley, R. D.; Davidson, E. C.; Chirik, P. J. A butadiene-derived semicrystalline polyolefin with two-tiered chemical recyclability. Chem 2024, 10, 1-15.
派遣先滞在期間
Date of Departure: 2024/8/1
Date of Return: 2024/10/1
国、都市等
Princeton, NJ, U.S.
機関名、受入先、会議名等
Chirik Group, Department of Chemistry, Princeton University
派遣中に学んだことや得られたもの
In the Chirik group, air- and moisture-sensitive organometallic catalysts are routinely handled using glovebox and high-vacuum line techniques. It was difficult to understand the detailed rules about these equipments in English, but I confirmed my concerns one by one. So, I learned how to use them well. When I was preparing for my experiment in the glovebox, A lab mate asked me to take her lab equipment in glovebox. I did it smoothly, then my lab mate said “Nice teamwork!” to me. It was one of my impressive events. I was so happy that I could communicate with the team well in English.
物理科学研究科 機能分子科学専攻 服部修佑

