October 30, 2023
[Press Release] Clocks of adaptive evolution run more slowly in ecological and geographical peripheries: The adaptation front equation explains how species flows in geographical timescale generate living fossils
Hiroshi Ito 1 , Akira Sasaki 1
1 Graduate University for Advanced Studies, SOKENDAI
Summary
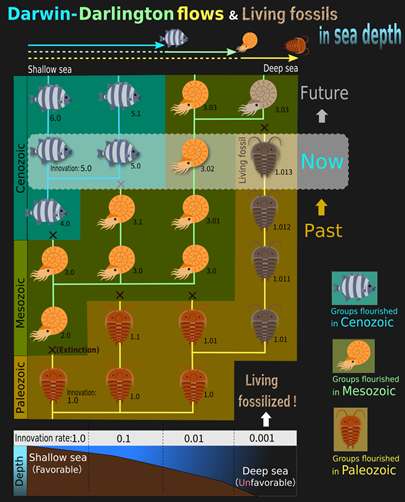
The evolutionary history of life can be viewed as the repeated rise and fall of various groups of organisms. Indeed, at various taxonomic levels, the fossil record shows repeated emergence of new groups that have achieved evolutionary innovations, followed by their diversification invading into wide ranges of ecological environments and exclusion of old indigenous groups (Figure 1).
The emergences of new groups have often occurred in biologically favorable environments (e.g., lowlands and shallow seas) and regions (e.g., tropics), leading Charles Darwin and other pioneering biogeographers to hypothesize that there exist evolutionary flows of species (or higher taxa) from favorable environments and regions to unfavorable ones.
In our present study, we have analyzed such evolutionary species flows by a newly developed equations and by evolutionary simulation analysis (Figure 2). As a result, we have clarified the factors that govern the direction and speed of the evolutionary flow and the net diversification rate (speciation rate minus extinction rate) in each environment or geographical region. Furthermore, we have shown that “living fossil” species, which persist for long time periods with their adaptive evolution almost ceased, emerge in environmentally and/or geographically the most peripheries, as an inevitable byproduct of the evolutionary flows.
Background
The evolutionary history of life has a nested structure of rise and fall, where newly emerged groups have diversified and replaced pre-existing old groups at various scales. A typical example is the replacement of metatherian mammals by eutherian mammals having placentas. Another typical example is the decline of gymnosperms accompanied with the diversification of angiosperms having overies. The acquisition of placentas (for eutherians) and overies (for angiosperms) are evolutionary innovations, also known as “key innovations.” Such innovations are thought to have given the new groups advantages over the old ones in wide ranges of ecological niches (e.g., food sizes, food types, temperatures, and activity times), and hence caused the massive turnovers.
Emergences of the new groups have often occurred in biologically favorable environments (e.g., lowlands and shallow waters) and regions (e.g., the tropics). Pioneering biogeographers including Charles Darwin and Philip Darlington believed that the repeated emergence of new groups in favorable environments and their diversification invading into unfavorable environments have been generating flows of species or higher taxa from favorable to unfavorable environments in geological spatio-temporal scale. We refer to such innovation-driven evolutionary flows as the “Darwin-Darlington flows,” after these pioneers.
In the presence of the Darwin-Darlington flows, one would expect that relatively newer taxonomic groups, among closely related groups existing at the same era, inhabit more favorable environments, whereas older groups inhabit less favorable environments. Indeed, even today, when comparing closely related multiple groups, older groups tend to inhabit higher latitudes than lower latitudes, higher altitudes than lower altitudes, deeper waters than shallower waters, and deeper caves than the surface, and they may be referred to as remnant species. Furthermore, the long-term fossil record of marine invertebrates shows evolutionary flows from shallow to deep sea, which explains why groups that emerged during the Cambrian often inhabit the deep sea (Figure 1).
The deep sea harbors many species that look similar to fossil species from hundreds of millions of years ago, and these are known as “living fossils.” They may be extreme cases of remnant species that arise as a byproduct of the Darwin-Darlington flows. The term “living fossils” was coined by Darwin in his book “The Origin of Species.” He wrote “Species and groups of species, which are called aberrant, and which may fancifully be called living fossils, will aid us in forming a picture of the ancient forms of life.” As he predicted, living fossils might help us to understand ancient lives. However, how living fossils emerge, why they persist over long periods of time, and how they embody ancient forms of life are still shrouded in mystery.
Research result
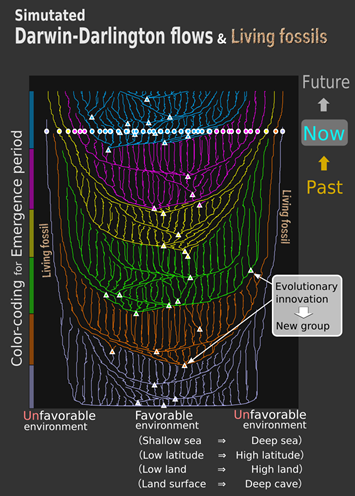
To study how living fossils can emerge and be maintained in the presence of the Darwin-Darlington flows, we have developed a new equation for analyzing the Darwin-Darlington flows. This equation, referred to as the adaptation front equation, describes the whole coevolutionary dynamics of a monophyletic species group in competitive relationship as a kind of fluid (more specifically, this equation is a hydrodynamic representation of the adaptive dynamics theory using the species packing theory). In this equation, we considered a niche space in which various ecological niches (i.e., environments) are aligned continuously along a one-dimensional axis (temperature, humidity, depth, elevation, etc.). In this space, species occupying the central niches with favorable environments tend to have large populations, whereas those occupying peripheral niches with unfavorable environments tend to have small populations. Then, species occupying the central niches produce mutant individuals more frequently than those occupying the peripheral niches, resulting in their faster adaptive evolution and hence their higher innovation rates. Consequently, species in the central niches repeat diversification and invasion into peripheral niches, outcompeting the indigenous species there. The outcompeted species go extinct or directionally evolve toward far more peripheral niches. These diversifications, extinctions, and directional evolutions together generate the Darwin-Darlington flows from the central to peripheral niches (Figure 2).
We reproduced the Darwin-Darlington flows by evolutionary simulation (Figure 2) and showed by the adaptation front equation that the directions and speeds of the flows (directional evolution), net diversification rates (speciation rates minus extinction rates), and divergence times (between closely related species) around each niche can be predicted from the distribution of innovation rates over the niche space. Particularly, in the most peripheral niches, the both of innovation rates and the Darwin-Darlington flows become significantly slow, as adaptive evolution of any species is significantly slow there. Therefore, species occupying the most peripheral niches become living fossils, which persist for long time periods with little evolutionary innovation and with deep divergence times from their closely related species (Figure 2).
These living fossils are destined to be eventually destroyed by species derived from new groups occupying the central niches. However, the adaptive evolution of these new species also slow down as they approach the most peripheral niches, so it will take a significantly long time before they destroy the living fossils. By that time, the once-new species themselves will have become living fossils and look conspicuously old-fashioned in comparison with the most innovative groups of the same era.
The above analysis corresponds to evolutionary dynamics in a single geographic region (e.g., a continent) with no migration barrier in it. We have also analyzed for multiple geographic regions by means of the adaptive front equation and evolutionary simulations. As a result, we have shown that living fossils emerge not only in the most peripheral niches but also in geographically the most peripheral regions (corresponding to Antarctica, Australia, the islands of New Zealand, etc.).
Implication
Biological interactions cause intrinsic and deterministic natures for the evolution of biological communities. However, it is not easy to examine how the interactions have been contributing to their evolutionary histories, since the most direct information source, the fossil record, primally tells us only the forms of past organisms.
The adaptive front equation we have constructed serves as a tool for converting hypotheses deduced from biological interactions into predictions testable in the fossil record, and hence would help us to understand the causality underlying the evolutionary history of life.
Authors
- Hiroshi Ito (The Graduate University for Advanced Studies, SOKENDAI, Research Center for Integrative Evolutionary Science, Postdoctoral researcher)
- Akira Sasaki (The Graduate University for Advanced Studies, SOKENDAI, Research Center for Integrative Evolutionary Science, Professor)
